To properly view the chemical formulas and expressions in this post you may need to download and install the RSC true type font on your computer. See the “Chemistry Font” page in the right sidebar.
The cathode is the positive electrode in an electrochemical cell. During discharge, its job is to absorb incoming electrons from the external electrical circuit by either taking cations (positive ions) in from the electrolyte or by emitting anions (negative ions) out into the electrolyte.
Nickel-cadmium and nickel-metal-hydride cells both use the same positive nickel-oxyhydroxide, NiOOH, cathode material to emit negative hydroxide anions, OHʇ, out into the electrolyte during discharge via the cathode reaction NiOOH + H¿O + eʇ ↓ Ni(OH)¿ + OHʇ. The outgoing OHʇ anions are dissolved into the electrolyte to be re-absorbed through oxidization at the negative anode electrode which consists of pure cadmium in a nickel-cadmium cell, forming cadmium-hydroxide via the anode reaction Cd + 2OHʇ ↓ Cd(OH)¿ + 2eʇ. In the case of a nickel-metal-hydride cell, the anode material is a metal-alloy-hydroxide, MH, that is oxidized back to just a metal-alloy and water via the anode reaction MH + OHʇ ↓ M + H¿O + eʇ.
Lead acid cells use a lead-dioxide, PbO¿, positive cathode material to absorb positive hydrogen cations, HÄ, in from the electrolyte and are major factors in the performance and life cycling of the lead acid cell. The lead-dioxide cathode is typically alloyed with 2-10 wt% of antimony or small amounts of calcium and other elements to help strengthen and improve the soft metal’s workability during manufacturing and to help improve its cycle life characteristics, especially for deep cycle applications. During discharge, the PbO¿ cathode is converted to lead-sulfate, PbSOÁ, through a two stage reaction, stage 1) PbO¿ + 4HÄ + 2eʇ ↓ PbÆÄ + 2H¿O, followed by stage 2) PbÆÄ + SOÁÆʇ ↓ PbSOÁ. The 4HÄ component of stage 1 and the SOÁÆʇ component of stage 2 are the products of breaking sulfuric acid molecules, H¿SOÁ, from the electrolyte by the reaction 2(H¿SOÁ) ↓ 4HÄ + 2(SOÁÆʇ). This is why the acidity of the aqueous based electrolyte decreases toward plain water during discharge.
The lead acid anode undergoes a similar two stage reaction during discharge by converting lead into lead-sulfate, a funny thing how the two active components in both the anode and cathode electrode materials both become lead-sulfate, PbSOÁ, during discharge.
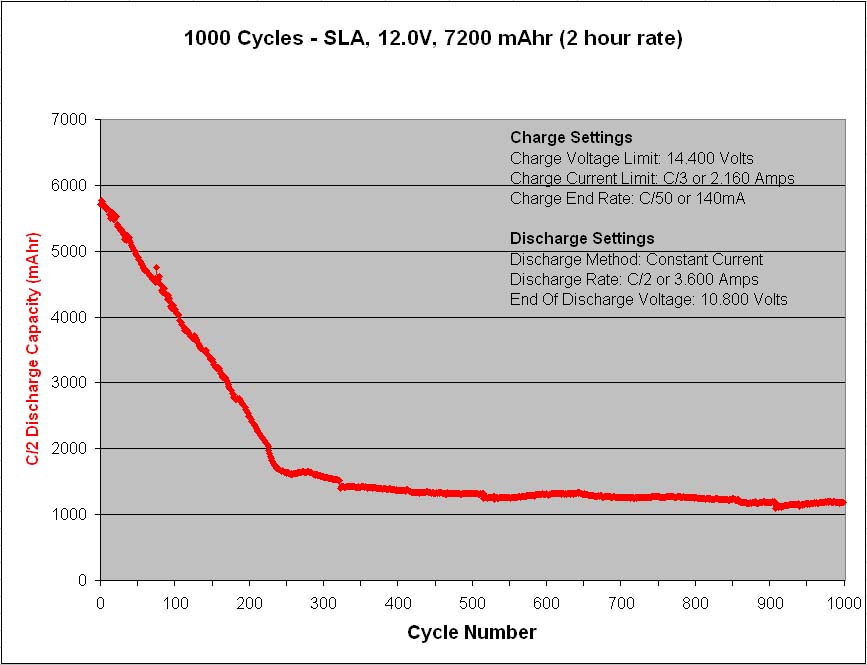
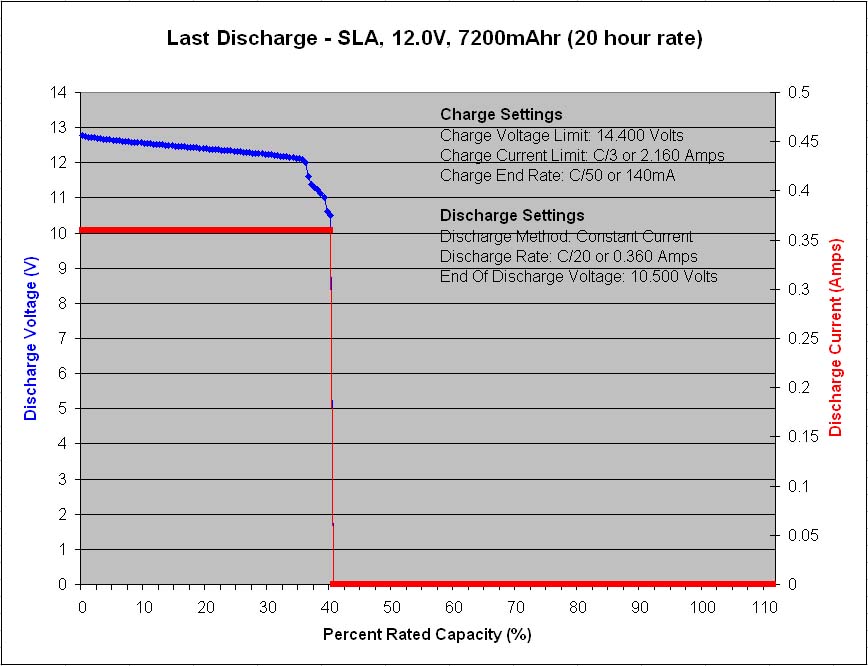
A major precaution for lead acid batteries is once discharged, they should never be left in the discharged state for any length of time longer than necessary because the lead-sulfate will slowly change from an amorphous structure to a crystalline structure, and once crystallized, the charge process will have great difficulty reversing the chemical reaction, as once the reversible constituents are locked up the battery loses its capacity to permanent damage. So always recharge a lead acid battery as soon as possible after any significant discharge occasion.
Also, the conversion rate of lead-sulfate from an amorphous structure to a crystalline structure increases with temperature, hence elevated temperature operating environments can be especially detrimental to lead acid type battery life.