A lithium 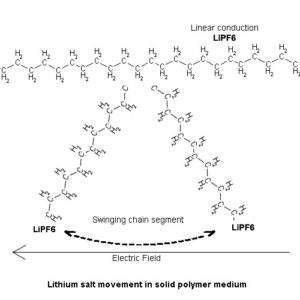 polymer battery is still a lithium ion battery except that it uses a solid plastic electrolyte material for ionic conduction between the anode and cathode electrodes rather than a liquid solvent. The solid polymer material still allows for movement of a dissolved lithium salt and ionic conduction between the electrodes while at the same time provides an insulating barrier to electronic conduction and physical separation of the positive and negative electrodes.
polymer battery is still a lithium ion battery except that it uses a solid plastic electrolyte material for ionic conduction between the anode and cathode electrodes rather than a liquid solvent. The solid polymer material still allows for movement of a dissolved lithium salt and ionic conduction between the electrodes while at the same time provides an insulating barrier to electronic conduction and physical separation of the positive and negative electrodes.
The main difference of a lithium polymer battery is the solid electrolyte’s solid properties and therefore methods by which movement of ionic molecules occurs within the material. There are two ways by which a lithium ion can propagate within the plastic electrolyte medium, one is by linear conduction along the axes of the polymer chains and chain segments, the other is by chain segment motion, bending or swinging action. In either case, the force for the movement results from the electric field interaction that exists between the cell’s electrodes and the charge on the free moving lithium cation.
Advantages of polymer electrolyte cell construction over liquid electrolyte cell construction are first and foremost the solid electrolyte itself is non-volatile and will not leak and therefore is less of a safety hazard in most applications. It can also be pressure laminated between the electrodes to provide a uniform solid mechanical contact between the electrodes that will not shift or move once assembled, in turn allowing for flat planar shaped cell designs and is not limited to cylindrical shaped packaging commonly used for liquid electrolyte systems. A further advantage then becomes the 100% packing factor and volumetric utility achievable with rectangular shaped cells for construction of multi-celled battery packs versus the use of cylindrical shaped cells that are theoretically limited to a volumetric utility of pi/4=78.5%.