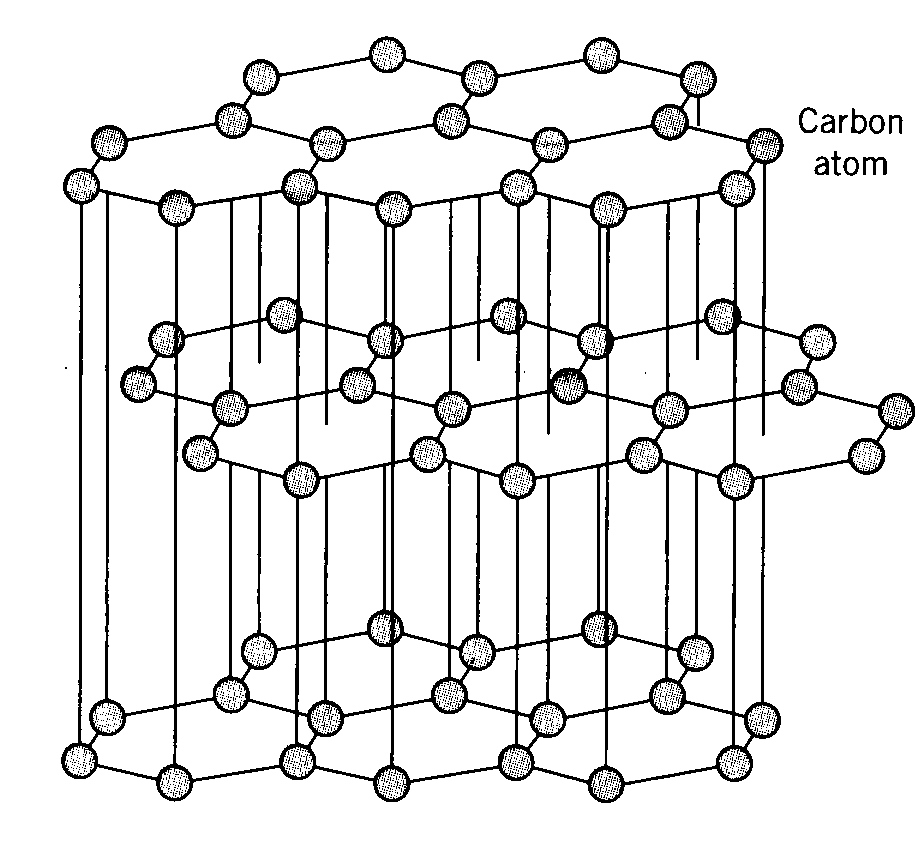
 The most common lithium ion anode material used today is carbon in its highly ordered graphite form with a maximum theoretical capacity of 372 mAh/g by the formation of LiC6. Graphite is the most thermodynamically stable form of carbon structure commonly referred to as the standard state where carbon atoms are held together by strong covalent bonding in a hexagonal lattice structure forming expansive sheets or layers loosely coupled together by much weaker van der Waals bonding between layers. The interlayer spacing provided by the weakly bonded graphene is highly accommodating to insertion and extraction of lithium cations. The graphene layers also have excellent electronic conductivity through the tightly bonded carbon planes providing low resistance electron pathways to facilitate easy electron pairing with lithium cations throughout the material. The graphite electrode’s structure is mechanically stable and highly cyclable undergoing very small volume change of only about 10% from fully lithiated to fully de-lithiated and can provide 1000’s of cycles without any appreciable loss of capacity.
The most common lithium ion anode material used today is carbon in its highly ordered graphite form with a maximum theoretical capacity of 372 mAh/g by the formation of LiC6. Graphite is the most thermodynamically stable form of carbon structure commonly referred to as the standard state where carbon atoms are held together by strong covalent bonding in a hexagonal lattice structure forming expansive sheets or layers loosely coupled together by much weaker van der Waals bonding between layers. The interlayer spacing provided by the weakly bonded graphene is highly accommodating to insertion and extraction of lithium cations. The graphene layers also have excellent electronic conductivity through the tightly bonded carbon planes providing low resistance electron pathways to facilitate easy electron pairing with lithium cations throughout the material. The graphite electrode’s structure is mechanically stable and highly cyclable undergoing very small volume change of only about 10% from fully lithiated to fully de-lithiated and can provide 1000’s of cycles without any appreciable loss of capacity.
Non-graphitic or disordered carbon contains the same covalently bonded hexagonal lattice structures as graphite but in much smaller pieces differing in size and orientation such that there’s no consistent expanse of ordered layering, similar to a sheet of tempered glass that’s been shattered into pieces and dumped into a pile. Amazingly, these disordered carbons can exhibit very high reversible capacities in the range of ~400 to ~2000 mAh/g. The exact mechanism for the higher than theoretical capacity over lithiated graphite LiC6 at 372 mAh/g is somewhat controversial but may be due to a collection of factors such as lithium being held in deposits on the carbon’s prismatic surfaces and edges or held in the carbon’s pores and surface defects or held in covalent Li2 formations similar to lithium metal deposits but not quite due to the surrounding carbon. Volume change in disordered carbons is small, less than 10%, due to the soft disordered nature and increased spacing between smaller carbon pieces. Problems with disordered carbons are they can exhibit higher voltage hysteresis than graphite between charge and discharge functions causing lower cyclic efficiency and heating, also their higher capacities are often obtained at only a few milli-Volts above Li+/Li increasing the risk of lithium metal plating and higher irreversible first charge capacity losses compared to graphite.
Overall, lithiated graphite electrodes are fantastic anode materials in lithium ion batteries with amazing abilities for graphene layers to operate super-efficiently at insertion, storage and removal of lithium cations in and out of the van der Waals spacings between layers. Highly ordered graphite is cheap to manufacture, light weight, abundant, non-toxic, environmentally friendly, has high safety, high capacity, high reversibility, high cyclability and low volume change upon lithium insertion/extraction, and operates at low potentials versus Li+/Li near 0.1-0.2 Volts. The only shortcomings of lithiated carbons are they have high irreversible first charge capacity losses and a double edge sword of low redox potentials versus Li+/Li that can at low temperatures result in lithium metal plating when charging due to slow insertion kinetics at low temperature. These problems can be managed and overcome by improvements in manufacturing processes, materials, electrolytes, additives, coatings and intelligent battery management systems.